General Web Resources
Drug Monographs and Assays Using Ion Chromatography
 In 2011, USP conducted a survey of the international pharmaceutical industry to evaluate existing procedures for the identification of ions and counterions in drug salts. After finding that spectrophotometric tests were the method most commonly being used, the USP undertook an initiative to modernize existing monographs across all compendia. Survey respondents preferred instrumental tests rather than traditional wet-chemistry tests, and expressed an interest in clarification of procedures and acceptance criteria. In addition to meeting these needs, modernizing the monographs will facilitate harmonizing tests with other pharmacopeias, and will enable more rigorous identification tests to decrease adulteration and counterfeiting of drugs.
In 2011, USP conducted a survey of the international pharmaceutical industry to evaluate existing procedures for the identification of ions and counterions in drug salts. After finding that spectrophotometric tests were the method most commonly being used, the USP undertook an initiative to modernize existing monographs across all compendia. Survey respondents preferred instrumental tests rather than traditional wet-chemistry tests, and expressed an interest in clarification of procedures and acceptance criteria. In addition to meeting these needs, modernizing the monographs will facilitate harmonizing tests with other pharmacopeias, and will enable more rigorous identification tests to decrease adulteration and counterfeiting of drugs.
Counterion Analysis
 Accelerate the determination and quantification of inorganic anions and cations in pharmaceutical formulations. Approximately half of all drugs are formulated and administered as salts. A broad selection of inorganic and organic ions can be used as drug substance counterions, with different ions being used to alter the biological and physicochemical properties such as solubility, stability, and dissolution rate. Screening and assaying of a broad variety of counterions and active pharmaceutical ingredients (APIs) is crucial for quality control (QC) of pharmaceutical salts to confirm identity of salt form and mass balance of the API.
Accelerate the determination and quantification of inorganic anions and cations in pharmaceutical formulations. Approximately half of all drugs are formulated and administered as salts. A broad selection of inorganic and organic ions can be used as drug substance counterions, with different ions being used to alter the biological and physicochemical properties such as solubility, stability, and dissolution rate. Screening and assaying of a broad variety of counterions and active pharmaceutical ingredients (APIs) is crucial for quality control (QC) of pharmaceutical salts to confirm identity of salt form and mass balance of the API.
Counter Ion Analysis Information
 With more than 50 % of pharmaceuticals on the market using counter ions, their analysis constitutes an essential part of drug development, QC and lot release processes to ensure patient safety and drug efficacy.
With more than 50 % of pharmaceuticals on the market using counter ions, their analysis constitutes an essential part of drug development, QC and lot release processes to ensure patient safety and drug efficacy.
Analyzing Pharmaceuticals by Ion Chromatography
 Most pharmaceuticals are produced synthetically in bulk then formulated into small dosage forms such as tablets or injectables. Many of these substances are manufactured in specific salt forms to promote solubility, stability, and bioavailability. The most common pharmaceutical counterions used in the development of basic drugs are chloride and sulfate.
Most pharmaceuticals are produced synthetically in bulk then formulated into small dosage forms such as tablets or injectables. Many of these substances are manufactured in specific salt forms to promote solubility, stability, and bioavailability. The most common pharmaceutical counterions used in the development of basic drugs are chloride and sulfate.
Antibiotics Analysis
 The USP monographs for some aminoglycoside drug substances (and drug products made from them) often involve high pressure anion exchange (HPAE) ion chromatography (IC) assays with integrated pulsed amperometric detection (IPAD). Chemical antibiotics are usually assayed by HPLC with UV detection, but where a chromophore is absent in either the active pharmaceutical ingredient (API) or impurities, IC with suppressed conductivity detection is considered the best alternative for selective determination.
The USP monographs for some aminoglycoside drug substances (and drug products made from them) often involve high pressure anion exchange (HPAE) ion chromatography (IC) assays with integrated pulsed amperometric detection (IPAD). Chemical antibiotics are usually assayed by HPLC with UV detection, but where a chromophore is absent in either the active pharmaceutical ingredient (API) or impurities, IC with suppressed conductivity detection is considered the best alternative for selective determination.
Glycan Analysis
 Biotherapeutic glycoproteins with complex glycosylation patterns have the potential to easily fall out of specification with changes in biomanufacturing processes. To meet regulatory demands such as ICH Q5E and ICH Q6B , manufacturers must carefully characterize glycosylation of proteins and its relation to the clinical activity of the medication. The complete analysis of a glycoprotein provides information on the primary structure of the oligosaccharides as well as their variation at individual glycosylation sites. See our complete solutions for glycan profiling of biotherapeutic proteins.
Biotherapeutic glycoproteins with complex glycosylation patterns have the potential to easily fall out of specification with changes in biomanufacturing processes. To meet regulatory demands such as ICH Q5E and ICH Q6B , manufacturers must carefully characterize glycosylation of proteins and its relation to the clinical activity of the medication. The complete analysis of a glycoprotein provides information on the primary structure of the oligosaccharides as well as their variation at individual glycosylation sites. See our complete solutions for glycan profiling of biotherapeutic proteins.
Glycan Analysis Information
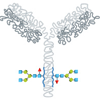 Glycans serve a variety of structural and functional roles in membrane and secreted proteins, with the majority of proteins undergoing some degree of glycosylation during their synthesis. Regulatory agencies worldwide, including the FDA and EMA, are increasing the demands placed upon manufacturers to comprehensively analyze the glycosylation of their therapeutics, and also to demonstrate how process can affect glycan composition.
Glycans serve a variety of structural and functional roles in membrane and secreted proteins, with the majority of proteins undergoing some degree of glycosylation during their synthesis. Regulatory agencies worldwide, including the FDA and EMA, are increasing the demands placed upon manufacturers to comprehensively analyze the glycosylation of their therapeutics, and also to demonstrate how process can affect glycan composition.
Speak to a Sales Specialist about Pharmacopoeial Modernization
 Thermo Fisher Scientific is here to assist you with technologies, workflows and methods for modern pharmaceutical analysis. Register today to receive that latest information to stay ahead of pharmacopeial modernization.
Thermo Fisher Scientific is here to assist you with technologies, workflows and methods for modern pharmaceutical analysis. Register today to receive that latest information to stay ahead of pharmacopeial modernization.
IC and Sample Preparation Cost Savings Calculator
 Discover the lowest total cost of ownership with Thermo Scientific Ion Chromatography and Sample Preparation instruments.
Discover the lowest total cost of ownership with Thermo Scientific Ion Chromatography and Sample Preparation instruments.









